Abstract
Background: Chronic lymphocytic leukemia (CLL) treatment (tx) has changed dramatically with the emergence of novel targeted therapies, and real-world data from observational studies continue to improve our understanding of tx patterns/outcomes. Beginning enrollment Oct 2015, informCLL (NCT02582879) began as a US, multicenter, prospective, observational real-world registry of patients (pts) with CLL who received CLL tx. From registry data, we describe demographic and clinical characteristics of pts with CLL, as well as tx patterns, dosing, and sequencing in clinical practice.
Methods: In the informCLL registry, 96% of recruiting sites across the US were community hematology-oncology sites. For this planned interim analysis (data cut: Feb 2018), txs were classified into 5 groups based on first tx at enrollment: chemoimmunotherapy (CIT), chemotherapy (CT), immunotherapy (IT), ibrutinib (ibr), and other novel agents. Eligible pts had to be ≥18 years (y), initiate tx of approved anti-CLL therapy within 30 days of enrollment, and provide consent. Categorical variables were summarized as frequency counts and percentages. Continuous variables were summarized as median, range, mean, and standard deviation.
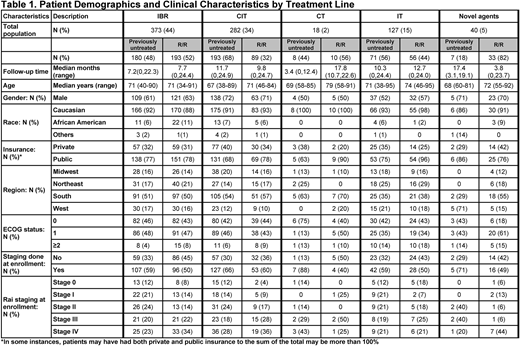
Results: 840 pts (previously untreated, n=459; relapsed or refractory [R/R], n=381) were enrolled. Median age was 70y (previously untreated=69y; R/R=71y). Most pts were male (64%), Caucasian (92%), and presented with ECOG PS 0/1 (previously untreated: 88%; R/R: 86%) (Table 1). 45% of previously untreated and 57% of R/R pts presented with Rai stage III/IV. Hypertension and type 2 diabetes were the most common comorbidities. At enrollment, the 3 most common txs were ibr (44%), CIT (34%), and IT (15%). In previously untreated pts, CIT was the most common tx (overall=42%; bendamustine+rituximab [BR]=20%, fludarabine+cyclophosphamide+rituximab [FCR]=10%, obinutuzumab+chlorambucil [GC]=9%) and single-agent ibr was the most common novel agent (38%). In R/R pts, single-agent ibr was the most common tx (48%). In pts <65y, CIT was the most common tx in previously untreated pts (overall=53%; BR=24%, FCR=19%, GC=6%) and ibr was the most common tx in R/R pts (55%). In pts ≥65y, ibr was the most common tx for previously untreated (43%) and R/R pts (49%). Median (range) follow-up was 9.4 months (0-24.9). Among previously untreated and R/R pts who received ibr at enrollment, 153/178 (86%) and 170/192 (89%) started once-daily doses of 420 mg, respectively. For pts receiving ibr at time of analysis, 19% had one or more dose modifications. In 96 previously untreated pts who received BR, 62 (65%) received <6 cycles (<6C), 14 (15%) received ≥6C, and 20 (21%) were still receiving tx. In 62 R/R pts who received BR, 38 (61%) received <6C, 5 (8%) received ≥6C, and 19 (31%) were still receiving tx. In pts who were no longer receiving therapy, median numbers of BR cycles for previously untreated and R/R pts were 5 and 4, respectively. In 44 previously untreated pts who received GC, 17 (39%) received <6C, 17 (39%) received ≥6C, and 10 (23%) were still receiving tx. In 12 R/R pts who received GC, 3 (25%) received <6C, 3 (25%) ≥6C, and 6 (50%) were still receiving tx. In pts who were no longer receiving therapy, median number of GC cycles for previously untreated and R/R pts was 5.5 each. In 45 previously untreated pts who received FCR, 31 (69%) received <6C, 4 (9%) received ≥6C, and 10 (22%) were still receiving tx. In 9 R/R pts who received FCR, 6 (67%) received <6C and 3 (33%) were still receiving tx. In pts who were no longer receiving therapy, median numbers of FCR cycles for previously untreated and R/R pts were 5 and 2.5, respectively. Following enrollment, 114/840 (14%) pts were treated with subsequent tx (previously untreated: 10%; R/R: 18%). Among these pts, 31/114 (27%) were initially treated with ibr. 43/114 (38%) pts subsequently received single-agent or combination ibr.
Conclusions: The most common agent used across all lines of tx was ibr. Most ibr-treated pts started at the FDA-recommended once-daily dose of 420 mg, with few dose interruptions/reductions. Most pts who received BR, FCR, or GC did not receive 6C of tx. Of pts who initiated subsequent tx, ibr was commonly used in pts who received IT, CIT, or CT. Few pts who received ibr switched to subsequent therapy. Continued follow up will further inform tx practices and enhance our understanding of CLL tx in the real-world setting.
Sharman:Pharmacyclics, an AbbVie Company: Consultancy, Research Funding; Acerta: Consultancy, Research Funding. Barrientos:Janssen: Consultancy; Gilead: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Pharmacyclics, an AbbVie Company: Consultancy, Research Funding. Brander:Teva: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; DTRM: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; BeiGene: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Acerta: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Novartis: Consultancy, Other: DSMB; TG Therapeutics: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; AbbVie: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Pharmacyclics, an AbbVie Company: Consultancy, Honoraria, Research Funding. Pagel:Gilead: Consultancy; Pharmacyclics, an AbbVie Company: Consultancy. Kadish:Janssen: Speakers Bureau; Celgene: Speakers Bureau; Takeda: Speakers Bureau; Pharmacyclics, an AbbVie Company: Speakers Bureau. Ghosh:Celgene: Consultancy; PCYC: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; F. Hoffman-La Roche Ltd: Research Funding; Pharmacyclics, an Abbvie Company: Consultancy, Research Funding, Speakers Bureau; Genentech: Research Funding; TG Therapeutics: Honoraria, Research Funding; Spectrum: Consultancy; Forty seven Inc: Research Funding; SGN: Consultancy, Research Funding, Speakers Bureau; Juno: Consultancy, Research Funding. Giafis:Pharmacyclics, an AbbVie Company: Employment, Other: Travel; AbbVie: Equity Ownership. Ipe:AbbVie: Equity Ownership; Pharmacyclics, an AbbVie Company: Employment, Other: Travel. Upasani:AbbVie (self and immediate family member): Equity Ownership; Pharmacyclics, an AbbVie Company (self and immediate family member): Employment. Sundaram:AbbVie: Employment, Equity Ownership, Other: Travel; Johnson & Johnson: Employment, Equity Ownership, Other: Travel. Ferrante:Janssen: Employment, Equity Ownership. Amaya-Chanaga:AbbVie: Equity Ownership, Other: Research performed while employed as an investigator of this study at UCSD. Review and approval of abstract performed while employed at Pharmacyclics, LLC, an AbbVie Company.; Pharmacyclics, an AbbVie Company: Employment, Other: Research performed while employed as an investigator of this study at UCSD. Review and approval of abstract performed while employed at Pharmacyclics, LLC, an AbbVie Company.. Iyengar:AbbVie: Equity Ownership; Pharmacyclics, an AbbVie company: Employment; Express Scripts: Patents & Royalties. Mato:Pharmacyclics, an AbbVie Company: Consultancy, Research Funding; AstraZeneca: Consultancy; Portola: Research Funding; Johnson & Johnson: Consultancy; Acerta: Research Funding; AbbVie: Consultancy, Research Funding; Prime Oncology: Honoraria; Celgene: Consultancy; Regeneron: Research Funding; Medscape: Honoraria; TG Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.